The Brigham Research Institute sponsors an annual Cores and Resources Fair which will feature research core facilities/resources, and provide an opportunity for the BWH research community to learn more about resources available to them as well as for cores to connect with researchers. This event is open to the entire BWH and MGB community – all researchers and research support staff who are interested in learning more about the prolific BWH, MGB, and Harvard core facilities are encouraged to attend.
For more information, please email bwhbri@partners.org.
The BRI is going bottled-water free! As it has been stated “more than 17 million barrels of oil are required to produce enough plastic water bottles to meet America’s annual demand for bottled water” and every year, 11 million metric tons of plastics enter our ocean. We hope as an institution and department to become more environmentally friendly. We encourage other departments to do the same! Please don’t forget to bring your filled reusable water bottles to all of our BRI events.
2024 Cores and Resources with Table Assignments
The Cores and Resources Fair will be held in the Hale Café Atrium. See details and contact for each resource by clicking on the dropdown box, and see below for layout map of the tables.
CONTACT
Sami Amr, PhD
The Biobank Genomics Core offers comprehensive support for translational and clinical sequencing and genotyping projects.
Our primary expertise is genomic sequencing and genotyping, including Next-Generation Sequencing (NGS) based on Illumina NovaSeq platform and array-based genotyping on Illumina’s Infinium platform. We offer whole genome, whole exome, and RNA-seq as well as sequencing for other applications and DNA genotyping for GWAS and EWAS from a variety of specimen types, including blood, saliva, and tissue blocks.
- Translational research in the clinical setting
- NGS-based sequencing projects
- End-to-end workflows: from extraction through analysis
CONTACT
Erin McKenna, MB, MS (she/her)
emckenna4@mgb.org
617-620-0557
Visit Website
Brigham Ignite is the Brigham’s early-stage innovation acceleration program focused on advancing discoveries with clinical and commercial potential by guiding researchers through the development process.
Along with supportive funding, we provide a team of experts in Licensing, Product, Development, Intellectual Property, and Commercialization designed and coordinated to help innovators take the first steps onto the translational path and beyond.
CONTACT
Chen Cao, MPH (he/him)
The Mass General Brigham Emerging Technologies and Solutions (MGBETS) aims to evaluate, pilot, and create outcomes around emerging digital technology in support of system strategy and missions. Supporting all of our hospitals and services across Mass General Brigham, MGBETS will focus in 3 areas:
- Accelerating clinical and operational pilots, with an initial focus on artificial intelligence;
- Building knowledge and awareness of emerging digital technologies; and
- Supporting MGB innovators building or leveraging early-stage digital technologies, in collaboration with MGB Digital Research Operations and MGB Innovation.
CONTACT
Steven Whitford, MS (he/him)
brac@partners.org
(617) 525-9246
Visit Website
The mission of the BRAC Laboratory is to provide a comprehensive menu of state-of-the-art high-quality research assays to the Partners and non-Partners research communities at competitive costs. The BRAC Laboratory is a CLIA certified laboratory and is accredited by the Joint Commission. We are also certified by the Centers for Disease Control’s Hormone Assay Standardization Program for many of our steroid hormone LC-MS/MS assays.
BRAC offers testing using immunoassay, and LC-MS/MS platforms. BRAC’s LC-MS/MS portfolio include novel tests such as: Total and Free Testo, GDF-11, GDF-8, DAKD, and DABk.
For more information, email brac@partners.org or visit https://brac-lab.bwh.harvard.edu/
CONTACT
Kevin Zinchuk, PharmD
kzinchuk@bwh.harvard.edu
(617) 525-3675
Visit Website
The Brigham and Women’s Hospital Investigational Drug Service (IDS) is division of the Department of Pharmacy Services and is devoted to the coordination of human drug research activities at Brigham and Women’s Hospital. This includes developing procedures to ensure timely and safe drug dispensing, maintaining inventory of investigational drugs, blinding and randomizing drug studies, serving as an information resource regarding investigational drugs and study protocols, meeting with principle investigators and study groups, participating in protocols as co-investigators and reviewing protocols as members of the Mass General Brigham Human Research Committee.
IDS acts as an informational resource for other Brigham and Women Hospital pharmacists and clinicians to assist in answering questions and solving dispensing issues with foreign drugs, orphan drugs, continued use of non-FDA approved agents from other institutions and emergency use investigational medications.
To comply with state, federal, accrediting bodies, and study sponsors; the BWH investigational drug services can support the following scenarios:
- Investigational new drugs being studied under an IND
- FDA approved drugs being studied under an IND or with IND exemption
- Drugs being studied under an Emergency IND
CONTACT
Polovna Laine
plaine@bwh.harvard.edu
cci@partners.org
The Center for Clinical Investigation (CCI) is a core service at Brigham and Women’s Hospital which offers extensive laboratory resources to investigators carrying out research. The CCI has three separate, fully equipped outpatient laboratories, as well as one fully equipped inpatient laboratory which is available to collect, process, ship, and store samples 24 hours a day 7 days a week. Each laboratory location houses both a refrigerated and room temperature centrifuge. Each location contains both a -80 freezer as well as a -20 freezer for short term sample storage. There is a 4C mini fridge available for short term storage as needed. The CCI also provides options for long term sample storage with two dedicated -80 freezers that are available to investigators upon request. All laboratory locations are fully stocked with dry ice and basic supplies which can be used for both sample processing and shipment as needed.
CCI lab facilities and processing equipment are available for study team use, however, the CCI can also provide personnel support with four experienced, certified phlebotomists on staff who can collect, process, store or ship samples as per the study team’s direction. In addition to simple processing, dedicated laboratory technicians also provide advanced support for PBMC processing as needed. At the inpatient lab facility CCI technicians can also support glucose analysis using YSI and Hemocue equipment.
CONTACT
Jun Case
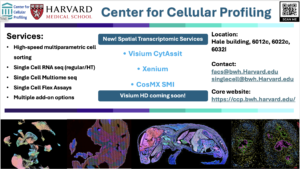
The mission of Center for Cellular Profiling (https://ccp.bwh.harvard.edu/) is to facilitate a disease deconstruction to disease reconstruction approach to understand cellular interactions in complex diseases. Our center provides comprehensive, end-to-end, single-cell and spatial transcriptomic genomic workflow to enhance the resolution of cellular profiling in diseases. Our facility is equipped with cutting-edge technologies, including droplet-based single-cell RNA sequencing by 10X Genomics and two BD AriaFusion high-speed cell sorters. Additionally, we feature the latest, single-cell resolution, spatial transcriptomics instruments including Xenium In Situ (10X Genomics), Visium CytAssist (10X Genoimcs), and CosMx Single Molecule Imager (Nanostring).
Our center provides comprehensive services to ensure successful experimental execution and high-quality data generation, encompassing consultation, project design, assay reagents, assay execution, troubleshooting, and data analysis. Our goal is to assist investigators in applying single-cell and spatial transcriptomic technologies to elucidate m cellular and molecular mechanisms of disease pathogenesis and uncover novel therapeutic targets.
We are located at Hale building for Transformative Medicine, Room 6012C, 6022C and 6032I. Please contact Jun Case (facs@bwh.harvard.edu; singlecell@bwh.harvard.edu) for further information.
CONTACT
Tim Janicki, MBA
tjanicki@bwh.harvard.edu
(617) 278-0553
Visit Website – Crimson Core
Visit Website – MHMC
The Crimson Core supports research studies and clinical trials, providing tens of thousands of remnant samples to area researchers and laboratories each year. Core services include the provision of discarded collars, remnant clinical samples, buffy coat processing, microbial isolate processing, CLIA testing, pharmacokinetic processing and storage for clinical trials, and prospective sample collection.
The Massachusetts Host-Microbiome Center promotes further understanding of host-microbiome interactions in health and disease, emphasizing a focus on function to define causative effects of the microbiota in vivo and to harness this knowledge in developing new therapies, diagnostics and further commercial applications. Core services include gnotobiotic study husbandry and processing, microbial and molecular processing, 16S phylotyping, genome sequencing, and project development.
CONTACT
Mike Peluso
mouseengineeringcore@gmail.com
(617) 432-6182
The Mouse Engineering Core, established in 1992 by Dr. Arlene Sharpe, provides gene targeting and microinjection services using state-of-the-art facilities and equipment.
The Core has extensive experience generating transgenic mice using DNA constructs (including BAC DNAs) and lentiviral constructs. We also offer CRISPR injections for DNA engineering in vivo. We have a track record of success generating KO/KI mice by performing gene targeting, or by expanding targeted ES cells from consortia.
The Core works with investigators to achieve optimal outcomes for each project.
CONTACT
Rie Maurer
The Harvard Catalyst Biostatistical Consultation program, funded by the National Institutes of Health, supports all Harvard postdoctoral and faculty investigators at Harvard schools and affiliated academic healthcare centers undertaking clinical and translational research. Drawing on a team of highly skilled biostatisticians from the Harvard academic medical and hospital community, the program offers biostatistics consultations and expertise on a range of relevant areas to researchers as they launch new clinical and translational projects. In addition, the program also offers bioinformatics consultations.
The program also provides training for clinical investigators in the principles and methods of biostatistics via our Postgraduate Education’s Applied Biostatistics Certificate course, as well as seminars, lectures, and workshops. In addition, the program promotes the intellectual and professional development of Harvard Catalyst Biostatisticians.
Biostatistics consultations focus on clinical and translational projects in the early stages of development. Typical consultations are between one to three hours, rarely exceed 10 hours.
Statistical services focus on clinical and translational projects in any stage of development. Typical consultations cover the following:
- Grant submission/resubmission, including Harvard Catalyst Pilot Funding
- IRB submission
- Protocol review
- Design for non-grant project/risibility consultation
- Analysis planning and advice
- Assistance with response to a manuscript or grant reviewer
- Assistance with ClinicalTrials.gov reporting
We also offer free weekly virtual office hours every Tuesday at 1:00 pm. Please contact Rie Maurer to request Zoom link (rmaurer@bwh.harvard.edu).
CONTACT
Richard Walsh, PhD
cryoem@crystal.harvard.edu
(617) 432-7042
The Harvard Cryo-Electron Microscopy Center for Structural Biology, founded in 2018, is a collaboration between Harvard Medical School, Dana-Farber Cancer Institute, Boston Children’s Hospital, and Massachusetts General Hospital, offering state-of-the-art Cryo-EM instrumentation and expertise through a team of six scientists. Our core mission is to enable researchers of all experience levels to efficiently incorporate structural determination as a powerful part of their scientific research. We strive to optimize research productivity while training users to become expert electron microscopists.
CONTACT
Vladimir Vrbanac, DVM (he/him)
vvrbanac@mgh.harvard.edu
(857) 268-7094
The HISMP provides an array of humanized mice, including BLT mice, useful for a broad array of investigations into human disease.
We are currently generating over 2,500 humanized BLT mice per year, of which approximately 2,300 demonstrate human immune reconstitution above our stringent criteria for use in experiments.
We routinely generate large batches of greater than 80 BLT humanized mice, and the HLA alleles, KIR alleles, and CCR5 alleles of each batch are genotyped.
Our group consequently has the capability to provide you with all the reconstituted BLT mice you need for your proposed work in this application.
Our program has all the resources required for generating, caring, and analyzing these animals. Our personnel are highly experienced in the microsurgical skills needed to transplant human immune tissues into these mice.
CONTACT
Jennifer Smith, PhD
jennifer_smith@hms.harvard.edu
(617) 432-5735
The ICCB-Longwood Screening Facility is a high-throughput small molecule and functional genomics core at HMS. There are over 500,000 compounds and natural product extracts available for screening, as well as siRNA, miRNA, and arrayed sgRNA libraries targeting the entire human and mouse genomes. Instruments available for experiments in microplate format include bulk reagent dispensers, automated pipettors, acoustic dispensers, high-content microscopes, plate readers, cytometers, and qPCR. In addition to high-throughput screening efforts, ICCB-L supports custom automation projects and makes equipment available to scientists conducting experiments in microplate format.
CONTACT
Paula Montero Llopis, PhD (she/her)
paula_monterollopis@hms.harvard.edu
(617) 432-0177
The Microscopy Resources On the North Quad (MicRoN) is an open access light microscopy core at NRB. We strive to educate and empower each trainee to accomplish their research goals, leading to enhanced rigor and reproducibility. MicRoN manages 14 instruments supporting a wide range of modalities including widefield, TIRF, spinning disk and single point scanning confocal, multiphoton, super-resolution, fluorescence lifetime imaging microscopy (FLIM), analysis and automated slide scanning (5-color widefield and color imaging) and high content imaging (widefield and confocal). We are incorporating STED and lightsheet imaging (cleared samples) soon. We support most applications including live cell imaging, FRAP, FRET, FLIM-FRET, multiplexing, 2D and 3D imaging and analysis and more.
CONTACT
Seth Goldman, PhD
seth_goldman@hms.harvard.edu
(617) 432-1361
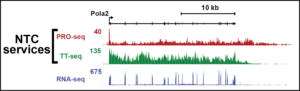
The mission of the Nascent Transcriptomics Core @ HMS is to offer the community a resource for the analysis of nascent transcription, providing new insights into gene regulation and enabling highly sensitive identification of regulatory regions such as enhancers. We offer a streamlined package of services allowing users to submit prepared cells and receive useful analyzed data. We also offer free consultations to discuss experimental and data analysis plans.
Analysis of nascent RNA–RNA that is actively being synthesized or within minutes of synthesis–can reveal changes in patterns of gene expression, transcription elongation or RNA processing and identify unstable transcripts (such as enhancer RNAs) that are challenging to detect by other RNA sequencing methods.
The core is happy to schedule consultations to help with experimental design, answer questions, and discuss data analysis.
Applications of nascent RNA analysis include:
- Pinpointing enhancers by identifying enhancer RNAs
- Define long non-coding RNAs expressed in a specific cell type or condition
- Identify sites of transcriptional initiation or pausing at single-nucleotide resolution
- Observe unstable RNA processing intermediates, and calculate rates of RNA processing or decay
We currently offer the following services:
PRO-seq: for sensitive measurement of nascent RNA production at genes and enhancers
- Single nucleotide resolution mapping of active RNAP across the genome
- Analysis of polymerase pausing and productive elongation
- Measurement of differential gene expression
- Identification of enhancers
TT-seq: a metabolic labeling assay using 4sU incorporation in living cells
- Monitors newly synthesized RNA species
- Sensitive and time resolved measurement of gene expression
- Can inform on RNA processing intermediates from splicing and 3′ end formation
CONTACT
Lai Ding, PhD
lding@bwh.harvard.edu
(781) 901-6116
The NeuroTechnology Studio (NTS) is a platform of advanced instrumentation and expert support for brain research at BWH. Its overall mission is to advance understanding of brain function and brain disease by providing Brigham researchers with access to cutting-edge technologies. The instrument fleet includes confocal (point and spinning disk), multiphoton, whole slide scanner, high-throughput imaging. he NTS will ensure that BWH remains at the forefront of these new developments. In addition to equipment, the Studio’s expert technical staff provide training workshops and individual consultation to users, enduring that they are able to use these powerful instruments to their full advantage.
CONTACT
Noel Palatas, MA (she/her)
visas@mgb.org
(857) 282-8211
GPS provides immigration services for Mass General Brigham Institutions, enabling access to talented professionals from all over the world. It is our goal to ensure regulatory compliance for international researchers and professional staff participating in Mass General Brigham programs. By facilitating compliance, GPS enables visiting professionals to focus on their work and their cultural exchange experience in the United States.
CONTACT
Amanda Higgins, MPH (she/her)
ahiggins@bwh.harvard.edu
(352) 228-1516
The mission of the PROVE Center is to expand the collection, analysis and utilization of patient-reported outcome measures (PROMs). The PROVE Center focuses on using innovative methods to study outcomes that matter the most to patients and their caregivers. Through collaboration with clinicians, researchers, patient advocates, health informatics experts and policymakers, we seek to amplify the patient voice in research, healthcare decision-making and patient care delivery.
The multidisciplinary PROVE Center team consists of clinicians and researchers with expertise in qualitative and quantitative methods relevant for PROM development, evaluation, and implementation.
Our team aims to promote and operationalize high-value and patient-centered care at MGB, throughout the United States, and around the world.